DEVELOPMENT AND EVALUATION OF MATRIX TYPE TRANSDERMAL PATCHES OF PIOGLITAZONE HYDROCHLORIDE
Keywords:
Pioglitazone hydrochloride, transdermal patches, in-vitro release, stability studies, TDDSAbstract
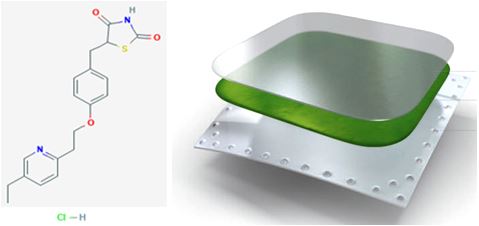
Objectives: Pioglitazone hydrochloride is a thiazolidinedione antidiabetic agent; it decreases insulin resistance which leads to decreased hepatic glucose output. It has short half-life, and is extensively metabolized and requires one to two times daily dosing. Present study aims to prepare transdermal patches of Pioglitazone hydrochloride to avoid all these drawbacks associated with it.
Methods: In present study, different transdermal patches of Pioglitazone hydrochloride were prepared using different polymers and evaluated on many parameters. Locally fabricated Franz diffusion cell was used for the in-vitro release study. Result revealed that there is a direct relationship with weight of the patch and drug content.
Results: The thickness lies in the range of 0.027 to 0.038mm. Average thickness was almost uniform within same formulation, a small variation in thickness was observed with different formulations. The weight of patches lies in the range of 43.31 to 46.3 mg. The percentage of the drug content lies in the range of 96.87 to 99.28. Content uniformity studies proved that the amount of Pioglitazone hydrochloride in each patch of 2.009 cm2 was found to be fairly uniform. Percent moisture absorption was found to be in the range of 4.388 to 5.465, largest in formulations of batch code T3 and least in the batch code T2.
Conclusion: The prepared transdermal drug delivery system of Pioglitazone hydrochloride using different polymers such as HPMC, EC, Chitosan and PVP had shown good promising results for all the evaluated parameters. However, for the in-vitro drug release and drug content result, formulation T4 was shown to be the optimized formulation, as higher percentage of drug release was obtained.
Peer Review History:
Article received on- 2 August; Revised 10 September; Accepted 14 October; Available online 15 November 2016
Academic Editor: Dr. Amany Mohamed Alboghdadly , Princess Nourah bint abdulrahman university, Riyadh, amalbgadley@pnu.edu.sa
, Princess Nourah bint abdulrahman university, Riyadh, amalbgadley@pnu.edu.sa
Reviewer(s) detail:
Dr. Maha Khalifa Ahmed Khalifa , Al-Azhar Universit - Cairo, Egypt, mahakhalifa.ahmed@hotmail.com
, Al-Azhar Universit - Cairo, Egypt, mahakhalifa.ahmed@hotmail.com
Dr. Viney Chawla , University Institute of Pharmaceutical Sciences, Baba Farid University of Health Sciences, Sadiq Road, Faridkot-Punjab 151203, drvineychawla@gmail.com
, University Institute of Pharmaceutical Sciences, Baba Farid University of Health Sciences, Sadiq Road, Faridkot-Punjab 151203, drvineychawla@gmail.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.