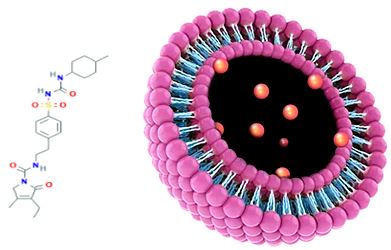
INVESTIGATION OF PRONIOSOMES GEL AS A PROMISING CARRIER FOR TRANSDERMAL DELIVERY OF GLIMEPIRIDE
Keywords:
Cholesterol, glimepiride, proniosomes gel, span 60, sustained release, transdermal drug deliveryAbstract
Objectives: The aim of the study was to develop a proniosomal carrier system that is capable of efficiently delivering entrapped glimepiride over an extended period of time for the treatment of type 2 diabetes.
Methods: Proniosomal gels were developed based on span 60 with and without cholesterol. The entrapment efficiency of drug inside niosomes developed from hydration of the proniosomes gel was also characterized. The in vitro release and skin permeation of glimepiride from various proniosomes gel formulations were investigated. The stability studies were performed at 4°C and at room temperature.
Results: The maximum entrapment efficiency was obtained when the cholesterol concentration was 10% of total lipid (90.02%). In vitro release through mixed cellulose ester membrane showed sustained release of drug from proniosomes gels. In vitro drug permeation across rabbit skin revealed improved drug permeation and higher transdermal flux with proniosomes gels compared to hydro-alcoholic gel of drug. Also, good physical stability was also achieved with proniosomes gels. Kinetics of in vitro skin permeation showed diffusion model of drug release from formulations.
Conclusion: The study proved that the concentration of cholesterol had great influence on the properties of proniosomes gels. Hence, proniosomes preparation containing 10% cholesterol can significantly increase trans-epidermal flux and prolong the release of glimepiride.
Peer Review History:
Article received on- 6 October 2016; Revised on- 10 November; Accepted on- 8 December; Available online 15 January 2017
Academic Editor: Dr. Asia Selman Abdullah , Al-Razi university, Department of Pharmacy, Yemen, asia_abdullah65@yahoo.com
, Al-Razi university, Department of Pharmacy, Yemen, asia_abdullah65@yahoo.com
Reviewer(s) detail:
Dr. Heba M. Abd El-Azim , Damanhour University, Egypt, h_m_abdelazim@hotmail.com
, Damanhour University, Egypt, h_m_abdelazim@hotmail.com
Dr. Mohamed Derbali , Faculty of Pharmacy, Monastir, Tunisia, mohamed.edderbali@gmail.com
, Faculty of Pharmacy, Monastir, Tunisia, mohamed.edderbali@gmail.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.