FORMULATION AND EVALUATION OF FAST DISSOLVING TABLETS OF ANTIEPILEPTIC DRUG
Keywords:
Bioavailability, epilepsy, fast dissolving tablet, lacosamide, pre-compression parametersAbstract
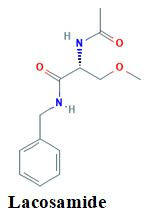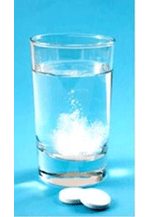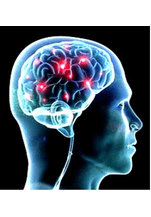
Objective: The objective of the present study was to prepare and evaluate the mouth dissolving tablet of lacosamide using super disintegrants like Guar Gum, and other excipients like microcrystalline cellulose and mannitol in different concentrations by direct compression method. Lacosamide has been shown to be an effective antiepileptic agent appropriate for epilepsy patients.
Methods: The mouth dissolving tablets were prepared by a single punch machine using a powder blend of super disintegrants and lacosamide. Post-compression parameters like hardness, weight variation, friability, in-vitro dispersion, drug content uniformity, and in-vitro drug release studies were carried out for all formulations.
Results: All formulations results were within official limits. Fast disintegration time obtained between 35sec. and 128 sec, was within the official limit. Different drug release kinetic models were applied for selecting batches for stability studies. These showed that there was no significant change in residual drug content in mouth dissolving tablets.
Conclusion: By the in-vitro disintegration, it is concluded that formulation prepared by Guar Gum (10%) showed faster disintegration time than that of MCC. This indicates that the use of super disintegrants increases the release of drug from the formulation. Therefore, it can be concluded that such mouth dissolving tablets are suitable delivery systems for lacosamide.
Peer Review History:
Received 2 October 2019; Revised 4 November; Accepted 29 December; Available online 15 January 2020
Academic Editor: Dr. Ali Abdullah Al-yahawi , Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
, Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
Reviewer(s) detail:
Dr. Iman Muhammad Higazy , National Research Center, Egypt, imane.higazy@hotmail.com
, National Research Center, Egypt, imane.higazy@hotmail.com
Dr. Mohamed Salama , Modern University for Technology & Information, Egypt, salama47@yahoo.com
, Modern University for Technology & Information, Egypt, salama47@yahoo.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.