VIRTUAL DESIGN OF NOVEL OF ORALLY BIOAVAILABLE PIPERAZINE INHIBITORS OF ENOYL-ACYL CARRIER PROTEIN REDUCTASE OF MYCOBACTERIUM TUBERCULOSIS WITH FAVORABLE PHARMACOKINETIC PROFILES
Keywords:
ADME, InhA inhibitors, Piperazine, pharmacophore, QSAR, tuberculosis, virtual screeningAbstract
Background: Drug-resistant strains have been a real problem for anti-tuberculosis therapies in recent decades. Here we elaborated the virtual rational design and evaluation of a novel class of piperazine (PPZ) analogs as InhA-Mt inhibitors with a favorable pharmacokinetic profile.
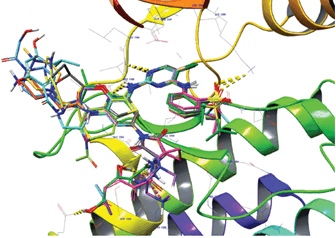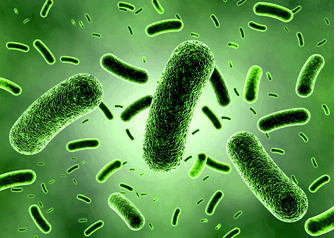
Method: By in situ modification of the crystal structure of InhA-PPZ1 (Protein Data Bank (PDB) entry code: 1P44), which is the reference structure of a test set of 12 PPZs with their known inhibitory potencies experimental data (IC50exp), we prepared three-dimensional (3D) models of Inha-PPZx complexes. A structure activity relationship (SQR) model was built in the gas phase in the search for active conformations of PPZ 1-12 linearly correlating the calculated enthalpy of formation of the InhA-PPZ complex and thepIC50exp. Finally Lipinski's rule of 5 was used to filter the VCL which was subsequently screened by the pharmacophore model. The predicted activities of the new PPZ analogs obtained were evaluated with the initial QSAR and their pharmacokinetic profile was also evaluated.
Results: The virtual combinatorial library of more than 310,550 PPZ analogs was filtered by Lipinski's rule until it reached 19,044 analogs. Virtual screening by the pharmacophore made it possible to select 50 potential new analogues with predicted inhibitory potencies up to 100 times better than that of PPZ1 (IC50exp=160 nM). The predicted pharmacokinetic properties of the new analogues showed high cell membrane permeability, side effects and high human oral absorption compared to current anti-TB candidates.
Conclusions: The combined use of molecular modeling and PH4 in virtual screening makes it possible to propose new powerful anti-tuberculosis drugs with favorable pharmacokinetic profiles.
Peer Review History:
Received 21 July 2024; Reviewed 13 September 2024; Accepted 20 October; Available online 15 November 2024
Academic Editor: Dr. Asia Selman Abdullah , Pharmacy institute, University of Basrah, Iraq, asia_abdullah65@yahoo.com
, Pharmacy institute, University of Basrah, Iraq, asia_abdullah65@yahoo.com
Reviewers:
 Antonio José de Jesus Evangelista, Federal University of Ceará, UFC, Brazil, tony_biomed@hotmail.com
Antonio José de Jesus Evangelista, Federal University of Ceará, UFC, Brazil, tony_biomed@hotmail.com
 Asmaa Ahmed Mohamed Ahmed Khalifa, Pharos University, Alexandria, Egypt, asmaa.khalifa@pua.edu.eg
Asmaa Ahmed Mohamed Ahmed Khalifa, Pharos University, Alexandria, Egypt, asmaa.khalifa@pua.edu.eg
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.