DEVELOPMENT AND ESTIMATION OF ANTI-INFLAMMATORY ACTIVITY OF TOPICAL ETORICOXIB EMULGEL BY CARRAGEENAN INDUCED PAW OEDEMA METHOD
Keywords:
Anti-inflammatory effect, carrageenan, emulgel, etoricoxib, gelling agent, hydrophobicAbstract
Objective: Emulgel is one of the emerging topical drug delivery system for the delivery of hydrophobic drugs which overcome various disadvantages of ointments and creams such as greasiness and phase inversion. Etoricoxib is poorly aqueous soluble Non steroidal Anti-Inflammatory Drug (NSAID). It is used in osteoarthritis, rheumatoid arthritis, acute gouty arthritis, ankylosing spondylitis, low back pain, acute postoperative pain, and primary dysmenorrheal. Its oral delivery is associated with greater chance of adverse effects or therapeutic failure and large amount of drug is lost in the vicinity of the target organ. Also oral administration of etoricoxib causes gastro-intestinal irritation. The aim of the present study was to develop an emulgel formulations using the gelling agent like Carbopol 934 and HPMC K4M with emulsifiers like Span 20 and Tween 20.
Methods: Six emulgel formulations were developed and evaluated on different parameters like physical appearance, pH, viscosity, extrudability, drug content, spreadability, in-vitro diffusion studies, and skin irritation test.
Results: All trials of emulgel were found to be homogeneous and smears were transparent without grittiness or presence of any particles. The pH values of emulgel batches were found in the range of 6.8-7.1 which is similar to skin pH.
Conclusion: Best formulation of batch EG1 was further evaluated for stability study and anti-inflammatory activity in carrageenan induced rat paw edema. Anti-inflammatory effect of formulation EG1 was compared with standard market product Indomethacin.
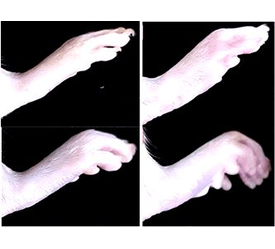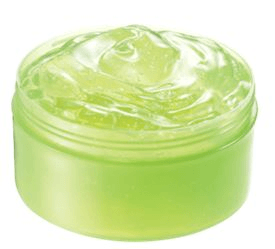
Peer Review History:
Received 6 April 2019; Revised 10 May; Accepted 25 June; Available online 15 July 2019
Academic Editor: Dr. Ali Abdullah Al-yahawi , Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
, Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
Reviewer(s) detail:
Dr. Sally A. El-Zahaby , Pharos University in Alexandria, Egypt, sally.elzahaby@yahoo.com
, Pharos University in Alexandria, Egypt, sally.elzahaby@yahoo.com
Dr. Evren Alğin Yapar , Turkish Medicines and Medical Devices Agency, Turkiye, evren.yapar@yahoo.com
, Turkish Medicines and Medical Devices Agency, Turkiye, evren.yapar@yahoo.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.