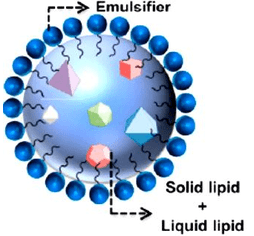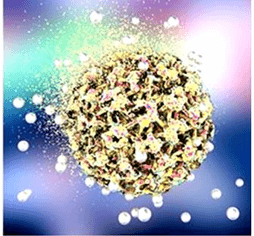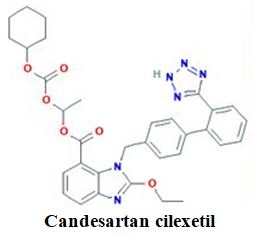
SCREENING STUDY FOR FORMULATION VARIABLES IN PREPARATION AND CHARACTERIZATION OF CANDESARTAN CILEXETIL LOADED NANOSTRUCTURED LIPID CARRIERS
Keywords:
Candesartan Cilexetil, encapsulation efficiency, in-vitro release, nanostructured lipid carriers, solid lipidAbstract
Objective: The current study inspects the screening of the formulation components further, evaluates the physicochemical properties of the nanostructured lipid carriers (NLCs) for the antihypertensive drug as Candesartan Cilexetil (CC). The sequence screening of all excipients required for the preparation of NLCs should be performed.
Methods: The prepared formulations were investigated for the different quality issues. The screening studies were performed to select the appropriate one of solid lipid, liquid lipid and surfactant. Also, investigation of physical compatibilities of solid lipid with liquid lipid and the ratios of them were evaluated. Furthermore, the physical characterization and quality issues of developed formulations were described and determined. Firstly, the solubility of CC in different solid and liquid lipids is the major parameter for the selection of the best one.
Results: Precirol® ATO 5, Compritol ® 888 ATO and Glyceryl Monostearate (GMS) were showed the maximum solubility of the CC (1000±4.12 mg, 1500±4.15 mg and 1750±3.16 mg), respectively. Hence, they were selected as the solid lipids for the development of NLCs. Liquid lipids Transcutol® HP (30±2.21 mg/ml), Labrasol® ALF (25±1.32 mg/ml) and CapryolTM 90 (18±1.34 mg/ml) were observed to have good affinity for the drug on systematic screening of different liquid lipids. All designed formulations observed in nanometer size of particles ranged from (408.9±11.5 to 114.6±8.3 nm) with high encapsulation efficiency around 99%.Also, the obtained results revealed that the ZP of the various formulations was consistently negative surface charge in between ((-13±2.3 to27.3±3.7 mV).
Conclusion: Finally, formula number nine of CC (CC-NLC9) which composed of GMS (solid lipid), CapryolTM 90 (liquid lipid) and Lutrol® F127: Cremophore® RH (surfactants combination) was selected as the best formulation after the rank order for further investigations in the next work. The current work clarifies a sequence steps for selection of excipients for NLCs by employing simple experiments.
Peer Review History:
Received 9 October 2019; Revised 7 November; Accepted 29 December; Available online 15 January 2020
Academic Editor: Ahmad Najib , Universitas Muslim Indonesia, Indonesia, ahmad.najib@umi.ac.id
, Universitas Muslim Indonesia, Indonesia, ahmad.najib@umi.ac.id
Reviewer(s) detail:
Dr. Mohammed Abdel-Wahab Sayed Abourehab , Umm Al-Qura University; Makkah Al-Mukarramah, Saudi Arabia, mohawahab2002@yahoo.com
, Umm Al-Qura University; Makkah Al-Mukarramah, Saudi Arabia, mohawahab2002@yahoo.com
Dr. Maha Khalifa Ahmed Khalifa , Al-Azhar Universit - Cairo, Egypt, mahakhalifa.ahmed@hotmail.com
, Al-Azhar Universit - Cairo, Egypt, mahakhalifa.ahmed@hotmail.com
Dr. Evren Alğin Yapar , Turkish Medicines and Medical Devices Agency, Turkiye, evren.yapar@yahoo.com
, Turkish Medicines and Medical Devices Agency, Turkiye, evren.yapar@yahoo.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.