ENHANCEMENT OF WOUND HEALING BY TOPICAL APPLICATION OF EPIDERMAL GROWTH FACTOR IN ANIMAL MODEL
Keywords:
Animal wound model, gene engineering, Recombinant human epidermal growth factor (rhEGF), wound healingAbstract
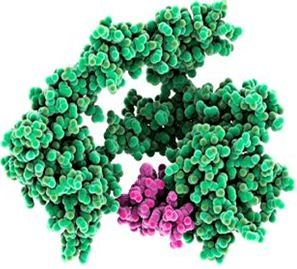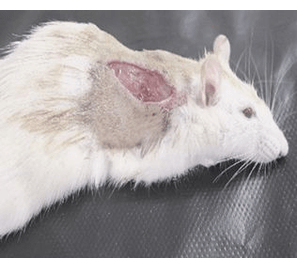
Objective: Wound healing is a complex process of biological events involving re-epithelialization and granulation that are mainly mediated by several endogenously released growth factors such as epidermal growth factor. This work was undertaken to study the effects of various doses of locally applied recombinant human epidermal growth factor (rhEGF) on wound healing in rats.
Methods: Recombinant human EGF consists of 53 amino acids. In vitro, rhEGF promoted its obvious cell growth and proliferation when added to cultured 3T3 cells using MTT assay. In the test groups, in vivo, wound sites were given daily with a solution containing 2, 5, 10, 50ug of EGF spray and 40ug of EGF ointment, respectively.
Results: Current study presented evidence that a significant decreased healing time in wound was observed in all rhEGF groups when compared with the control, and reach to its maximal efficacy at 10ug/ml of rhEGF spray. The rate of wound closure was over 50% at initial 3 days of treatment. Treatment with rhEGF significantly decreased the length of time to over 50% healing by approximately 4-5 days, and that to 70% and 90% healing by approximately 3-4 days and 3 days, respectively. A stimulatory, dose-dependent effect of EGF on wound healing was observed with increased hEGF concentration. In toxicological group, higher doses of 100ug/ml of rhEGF spray was applied by local dorsal incision in rats. Moreover, a dose of single 200ug, single 300ug or 300ug within 24 hrs of subcutaneous and intramuscular rhEGF injection was given respectively. There were no significant adverse side effects.
Conclusion: Current study recommended a proposal of clinical drug doses in wound at 2µg, 5 µg and 10 µg /ml of rhEGF spray, and 10 µg and even higher 40 µg rhEGF/g of ointment. The results indicated that prepared rhEGF by genetic engineering in current study is safe, and is emerging in clinical effective use in assisting wound healing time.
Peer Review History:
Received 9 December 2019; Revised 15 January 2020; Accepted 26 February; Available online 15 March 2020
Academic Editor: Dr. Ali Abdullah Al-yahawi , Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
, Al-Razi university, Department of Pharmacy, Yemen, alyahawipharm@yahoo.com
Reviewer(s) detail:
Asmaa Ahmed Mohamed Ahmed Khalifa , Pharos University, Alexandria, Egypt, asmaa.khalifa@pua.edu.eg
, Pharos University, Alexandria, Egypt, asmaa.khalifa@pua.edu.eg
Dr. Sabah Hussien El-Ghaiesh  , Tanta University, Egypt, s.ghaiesh@gmail.com
, Tanta University, Egypt, s.ghaiesh@gmail.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.