PREPARATION AND CHARACTERIZATION OF TOLTERODINE TARTRATE PRONIOSOMES
Keywords:
Tolterodine tartrate, proniosome, Span 60, Tween 40, in vitro releaseAbstract
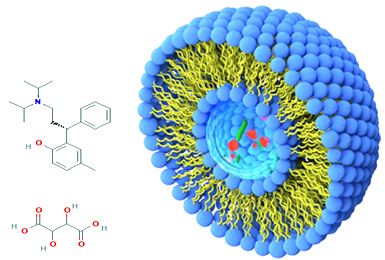
Objective: Proniosomes are dry free flowing, granular products that are water soluble carrier particles, coated with surfactants and can be hydrated to form niosomal dispersion immediately before use in hot aqueous media. Tolterodine, is medication used to treat frequent urination, urinary incontinence, or urinary urgency. It acts on M2 and M3 subtypes of muscarinic receptors.
Methods: Tolterodine tartrate proniosome formulations were prepared by coacervation phase separation method by using different surfactants in different ratios. The prepared proniosomal formulations were evaluated for vesicle size, rate of spontaneity, encapsulation efficiency, drug content, In vitro release study and stability studies.
Results: The size range was found to be 15.28±0.33 to 16.43±0.22 µm. Viscosity of all formulations lies in the range of 7244-9314 cp. Maximum release was shown by formulations of batch PG4 (87.45 %), and minimum for formulations of batch PG4 (50%), after 12 h. Optimized formulations PG4 shows stability at different temperatures.
Conclusion: Tolterodine tartrate proniosome formulations prepared by using coacervation phase separation method are capable of releasing drug for the extended period of time.
Peer Review History:
Received 9 February 2017; Revised 7 March; Accepted 26 April, Available online 15 May 2017
Academic Editor: Dr. DANIYAN Oluwatoyin Michael , Obafemi Awolowo University, ILE-IFE, Nigeria, toyinpharm@gmail.com
, Obafemi Awolowo University, ILE-IFE, Nigeria, toyinpharm@gmail.com
Reviewer(s) detail:
Dr. Mohammed Abdel-Wahab Sayed Abourehab , Umm Al-Qura University; Makkah Al-Mukarramah, Saudi Arabia, mohawahab2002@yahoo.com
, Umm Al-Qura University; Makkah Al-Mukarramah, Saudi Arabia, mohawahab2002@yahoo.com
Dr. Sally A. El-Zahaby , Pharos University in Alexandria, Egypt, sally.elzahaby@yahoo.com
, Pharos University in Alexandria, Egypt, sally.elzahaby@yahoo.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.