COMPARATIVE STUDY OF THE EFFICACY OF STEM CELLS IN CORNEAL REGENERATION IN A CHEMICAL BURN IN RABBITS
Keywords:
Chemical burn, corneal regeneration, limbic deficit, stem cell, transplantationAbstract
Objectives: This study compares the efficacy of stem cell transplantation in corneal regeneration and restoration of the limbic deficit in an experimental chemical burn in rabbits.
Methods: Biopsy was performed of the limbus and the chemical burns for all rabbits, and we collected the amniotic membranes from a pregnant female rabbit. We kept a control group without transplantation, to study spontaneous and natural healing, and we transplanted the stem cells produced in vitro under the corneal epithelium burned. To compare the result, we tested a group for amniotic stem cell transplantation, a group for limbal stem cell graft, and another group for combined transplantation of both types of stem cells.
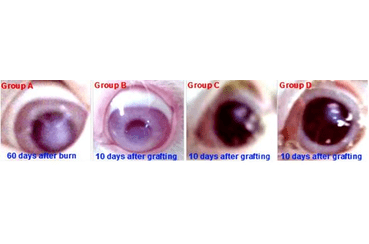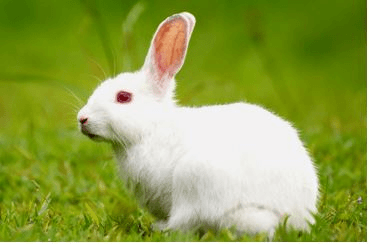
Results: Transplanted rabbits develop permanent unilateral blindness due to a severe limbic deficit. The group receiving only amniotic stem cells shows temporary anatomical improvement without functional recovery. The two groups receiving limbal stem cells alone or combined with amniotic stem cells showed anatomical and functional satisfaction with quick recovery time for the combined transplantation.
Conclusions: A simple chemical burn can establish permanent blindness. When the limbic deficit is important, spontaneous healing is not available. Transplantation of stem cell transplant is the only way to repair this deficit and regenerate the cornea. Only limbic stem cells can be sufficient. Amniotic stem cells can support and speed up the healing time when it combined to limbal stem cells graft.
Peer Review History:
Received 5 June 2020; Revised 11 July; Accepted 23 August; Available online 15 September 2020
Academic Editor: Essam Mohamed Eissa , Beni-Suef University, Egypt, dressamceutics@yahoo.com
, Beni-Suef University, Egypt, dressamceutics@yahoo.com
Reviewer(s) detail:
Dr. Mohamed Amin El-Emam , Department of Pharmacology and Therapeutics, Faculty of Pharmacy and Drug Manufacturing, Pharos University in Alexandria (PUA), Alexandria, Egypt, mohamed.elemam@pua.edu.eg
, Department of Pharmacology and Therapeutics, Faculty of Pharmacy and Drug Manufacturing, Pharos University in Alexandria (PUA), Alexandria, Egypt, mohamed.elemam@pua.edu.eg
Francesco Ferrara ,USL Umbria 1, Perugia, Italy, francesco.ferrara@uslumbria1.it
,USL Umbria 1, Perugia, Italy, francesco.ferrara@uslumbria1.it
Maged Almezgagi , Department of Immunology, Medical College of Qinghai University, Qinghai Xining 810001, China, 1902244017@qq.com
, Department of Immunology, Medical College of Qinghai University, Qinghai Xining 810001, China, 1902244017@qq.com
Dr. Asia Selman Abdullah , University of Basrah, Iraq, asiaselman2016@gmail.com
, University of Basrah, Iraq, asiaselman2016@gmail.com
Downloads

Published
How to Cite
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









 .
.